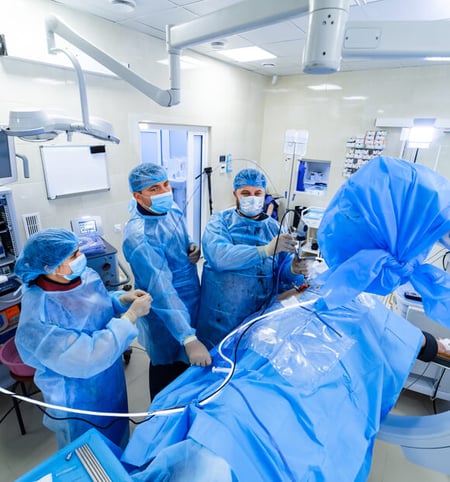
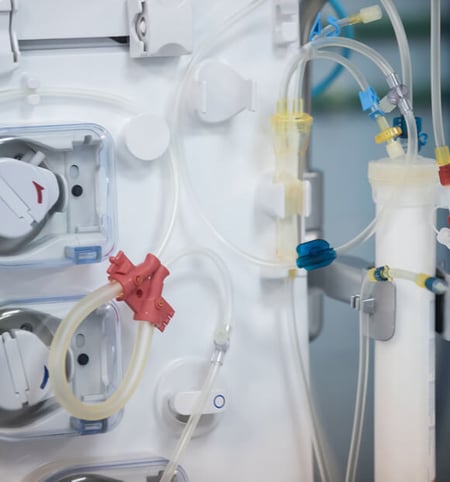
A seal for every medical device use case
Whether your product is a predicate improvement or a novel Class II or Class III device we can help you find the best seal or media manifold/separator design.
If you are in the prototype phase we can quickly help iterate when your seal element layout needs to change. We have strong relationships with fast turn LSR molding houses and access to premium USP Class VI silicone elastomer formulations.
From well-established profiles like o-rings, x-rings, quad rings, thru lip seals, check valves and complex molded shapes. When you’re ready to ramp, our molders can scale quickly too, giving you plenty of time to validate parts from higher cavity count tooling.
Seals are important no matter the application. Putting in the wrong size and type of seal will negatively impact your application. An incorrectly sized seal will introduce a leak path, which could affect sterility of the product or waste of material if product is coming out of the application.
The wrong type of seal can also influence how robust the application is. If a product requires low press sealing, but a high-pressure seal is chosen, the seal will not be able to provide the necessary seal. This in turn would lead to a leak path and would affect the application's performance.
.jpg?width=618&height=412&name=shutterstock_1019673052%20(1).jpg)